Trending Stories
Revolutionary Alzheimer’s Drug Leqembi Set to Receive Full FDA Approval

Revolutionary Alzheimer’s Drug Leqembi Set to Receive Full FDA Approval
Medicare Coverage Expansion Expected to Benefit Millions
In a groundbreaking development, the US Food and Drug Administration (FDA) is poised to grant traditional approval to Leqembi, an Alzheimer’s drug that has demonstrated the ability to slow the progression of the devastating disease.
This historic decision, expected to be announced on Thursday, marks the first time a medication has been proven to have a positive impact on the memory-robbing effects of Alzheimer’s.
A Paradigm Shift: Leqembi’s Potential Impact
Once the FDA grants its approval, the Centers for Medicare and Medicaid Services (CMS) will also revise its coverage policy, expanding access to Leqembi for an estimated one million individuals suffering from early-stage Alzheimer’s.
Developed by Eisai and Biogen, Leqembi received accelerated approval in January based on evidence of its ability to clear amyloid plaque buildups in the brain, which are closely associated with the disease.
However, due to a prior coverage decision by CMS, the drug has been underutilized. With a price tag of $26,500 before insurance coverage, many individuals have been unable to afford it.
Medicare Coverage Expansion Expected to Benefit Millions
Joe Montminy, a 59-year-old diagnosed with younger-onset Alzheimer’s in his early 50s, expressed the frustration of having limited access to the treatment due to insurance coverage issues.
The expansion of coverage is of great significance to him and others in similar situations, as it provides hope for effective treatment options.
A New Era of Treatment
Leqembi’s approval is currently limited to individuals with early forms of Alzheimer’s, specifically those with mild cognitive impairment or mild dementia who have confirmed amyloid plaques in their brains.
Dr. Lawrence Honig, a neurology professor at Columbia University Irving Medical Center, estimates that this group represents about one-sixth of the more than six million Americans currently diagnosed with Alzheimer’s.
However, it’s important to note that individuals with more advanced stages of the disease may not experience the same benefits from the drug and could face increased safety risks.
Dr. Honig emphasizes that while Leqembi represents a significant breakthrough, it is not a cure. In an 18-month clinical trial, the drug showed a 27% reduction in cognitive decline and functional impairment.
Dr. Honig believes that these treatments are just the beginning of a new era in Alzheimer’s research, expressing optimism for the development of more efficacious therapies in the future.
Balancing Potential Benefits and Risks
Leqembi, like any medication, comes with potential side effects and requires regular brain imaging for monitoring purposes.
During the trial, approximately 13% of participants experienced brain swelling or bleeding, and certain groups, such as those with specific genetic factors or individuals on blood-thinning medications, may face higher risks.
Healthcare systems have been preparing for the anticipated increase in Leqembi usage. Dr. Georges Naasan, the medical director of the Division of Behavioral Neurology and Neuropsychology at Mount Sinai, acknowledges the complexities involved in the process but affirms that preparations have been underway to ensure a smooth transition. Infusion centers, such as Vivo Infusion, are gearing up to handle the potential surge of new patients.
Medicare Coverage Expansion and Potential Implications
CMS has announced plans to broaden coverage for Leqembi if it receives traditional FDA approval. However, there are certain qualifications attached to this expanded coverage.
Medicare will cover approved drugs when physicians and clinical teams participate in the collection of real-world evidence through a registry.
This approach aims to assess the effectiveness of the medication in broader community practice. CMS will facilitate a portal where providers can submit the collected evidence free of charge.
While CMS’s approach has been met with some concerns from patient groups and the pharmaceutical industry, who argue that it may create barriers to treatment, the expanded Medicare coverage of Leqembi and similar drugs that slow the progression of Alzheimer’s is expected to have a significant impact on healthcare spending.
An analysis by KFF (formerly the Kaiser Family Foundation) indicates that if 10% of the estimated 6.7 million older adults eligible for the drug opt to take Leqembi, with an annual list price of $26,500, it could result in a spending increase of $17.8 billion.
This figure surpasses the total spending on the top 10 Part B drugs administered in doctors’ offices in 2021. Higher Medicare Part B premiums for all enrollees may be a consequence of the increased spending. Nevertheless,
Trending Stories
Sister Regina Liu: Empowering Health Through Acupuncture

Sister Regina Liu: Empowering Health Through Acupuncture
In the bustling world of healthcare, Sister Regina Liu stands out as a beacon of holistic healing. Her journey into the world of acupuncture is not only inspiring but also transformative for the countless individuals she has treated.
Through her dedication, Sister Regina has brought traditional Chinese medicine to the forefront, offering an alternative and complementary approach to modern medical practices.
The Journey of Sister Regina Liu
Sister Regina Liu’s path to becoming a renowned acupuncturist began with her deep-rooted interest in holistic health. Born into a family that valued traditional Chinese medicine, Sister Regina was exposed to the benefits of acupuncture from a young age. Her early fascination turned into a lifelong passion as she pursued formal education and training in the field.
Acupuncture: Bridging Ancient Wisdom and Modern Health
Acupuncture, a practice with origins in ancient China, involves inserting thin needles into specific points on the body to balance the flow of energy or “qi.” Sister Regina Liu has mastered this ancient art, using it to address a wide range of health issues.
From chronic pain to stress management, her expertise has provided relief to many who had exhausted conventional treatment options.
Impact on Community Health
Sister Regina’s impact extends beyond individual treatments. She has been instrumental in educating the community about the benefits of acupuncture, breaking down misconceptions, and making the practice more accessible.
Her workshops and seminars have enlightened many about the holistic approach to health, emphasizing the interconnectedness of body, mind, and spirit.
Success Stories and Testimonials
The success stories of Sister Regina’s patients are a testament to her skill and dedication. Many individuals who had lost hope found solace in her treatments.
For instance, Maria, a long-time sufferer of migraines, experienced significant relief after just a few sessions with Sister Regina. Her story is just one of many that highlight the transformative power of acupuncture under Sister Regina’s care.
Challenges and Triumphs
Like any journey, Sister Regina’s path was not without challenges. Integrating acupuncture into mainstream healthcare faced resistance initially.
However, her perseverance and the undeniable results of her treatments gradually won over skeptics. Today, Sister Regina is not only respected in the field of acupuncture but also in the broader medical community.
The Science Behind Acupuncture
While acupuncture is rooted in ancient practices, modern science has begun to unravel the mechanisms behind its effectiveness. Studies have shown that acupuncture can stimulate the release of endorphins, the body’s natural painkillers, and improve blood circulation.
These scientific validations have further cemented acupuncture’s place in contemporary healthcare, thanks in part to advocates like Sister Regina Liu.
Acupuncture in Modern Healthcare
Sister Regina’s work exemplifies how traditional practices can complement modern medicine. Hospitals and clinics increasingly incorporate acupuncture into their treatment plans, recognizing its benefits in pain management, mental health, and overall well-being. This integration signifies a broader acceptance and understanding of holistic health practices.
Future Vision
Looking ahead, Sister Regina Liu envisions a future where acupuncture and traditional Chinese medicine are fully integrated into the global healthcare system. She continues to advocate for research, education, and policy changes that support the inclusion of holistic practices in mainstream medicine.
How to Get Started with Acupuncture
For those new to acupuncture, Sister Regina offers practical advice on getting started. She recommends finding a certified acupuncturist, understanding the treatment process, and maintaining an open mind. Her guidance helps demystify acupuncture, making it more approachable for newcomers.
Conclusion
Sister Regina Liu’s journey in empowering health through acupuncture is a remarkable tale of dedication, resilience, and success. Her contributions have not only alleviated individual suffering but also enriched the broader understanding of holistic health. As acupuncture continues to gain recognition, Sister Regina’s legacy will undoubtedly inspire future generations of healers.
FAQs
1. What conditions can acupuncture treat?
Acupuncture can address various conditions, including chronic pain, migraines, stress, anxiety, digestive issues, and more. It is also used to support overall wellness and balance.
2. Is acupuncture safe?
Yes, when performed by a certified and experienced acupuncturist, acupuncture is safe. It involves using sterile, single-use needles and adhering to proper hygiene practices.
3. How many sessions are needed to see results?
The number of sessions varies depending on the condition and individual response. Some may experience relief after one session, while others may need multiple treatments.
4. Does acupuncture hurt?
Acupuncture needles are very thin, and most people feel minimal to no discomfort. Some may feel a slight tingling or warmth at the needle site.
5. How do I find a qualified acupuncturist?
Look for acupuncturists who are certified by recognized professional organizations and have positive patient reviews. Personal recommendations and consultations can also help in making an informed choice.
References
Trending Stories
In 2 Shape Gym Unveils Major Expansion in Stourport
Trending Stories
9 Reasons Why In-Person Friendships Are Irreplaceable
-

 Trending Stories1 year ago
Trending Stories1 year agoCDC: 1 in 4 Americans Still COVID-Free by End of 2022
-

 Health5 years ago
Health5 years agoMeghan Trainor Shares Motivational New Song ‘Blink’
-
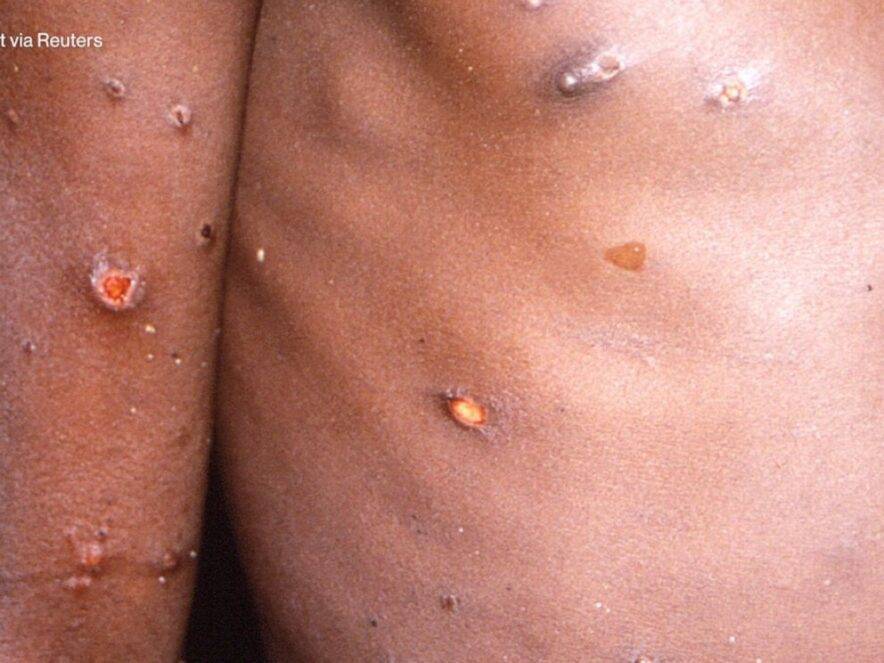
 Health2 years ago
Health2 years agoHow Long Does Monkey Pox Last Before It Surfaces in the Body?
-

 Health2 years ago
Health2 years agoWhat Causes Swollen Body? Understanding Edema and its Triggers
-

 Health3 years ago
Health3 years agoNutrition and the Importance of a Fitness Program – 3 Things to Know
-

 Health3 years ago
Health3 years ago5 Weird Reasons Why Pimples Disappear After Marriage
-

 Health3 months ago
Health3 months agoHow Do Pawpaw Seeds Support Cardiovascular Health?
-

 Health2 years ago
Health2 years agoHealth Benefits Of Pawpaw Seed? 7 Things To Know