Trending Stories
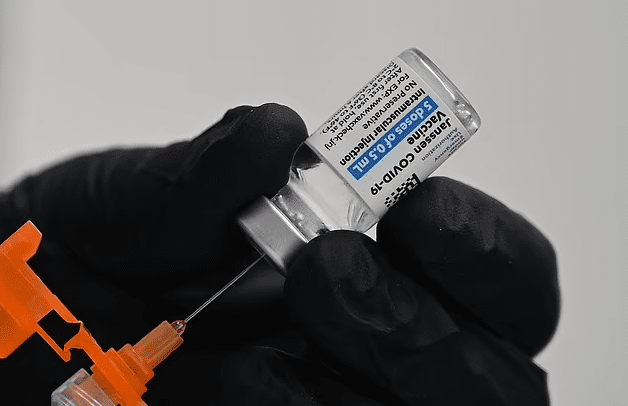
FDA Revokes Authorization of J&J’s COVID-19 Vaccine Due to Blood Clot
Last Updated on June 10, 2023 by Nurse Vicky
FDA Revokes Authorization of J&J’s COVID-19 Vaccine Due to Blood Clot Concerns and Reduced Demand
The FDA has decided to retract the authorization of the Johnson & Johnson (J&J) COVID-19 vaccine. This decision was due to reports of rare cases of blood clotting and a significant decline in demand for the vaccine. The development comes after approximately 19 million Americans have already received the J&J vaccine.
Background:
The controversy surrounding J&J’s COVID vaccine began early on due to health concerns and reports of contamination, which caused the public’s trust in the vaccine to diminish considerably. Notably, these concerns led to a brief suspension in 2021 following the reporting of severe blood clots in vaccine recipients.
Details on the FDA’s Decision:
Johnson & Johnson’s parent company, Jannsen, requested that the FDA withdraw the vaccine’s authorization when it was disclosed that the remaining 12.5 million doses had expired.
This was not surprising, as approximately 231 million Americans had already received either a single J&J shot or two mRNA vaccine doses from Pfizer or Moderna.
As vaccination fatigue has started to affect the U.S., and considering the challenges faced by J&J’s single-dose vaccine, including production problems and health concerns, the pharmaceutical company has decided to step back from the COVID-19 vaccine field.
The issues faced early in the vaccine’s life, such as the disposal of about 60 million doses due to manufacturing difficulties, have further complicated J&J’s situation.
Comparative Analysis of Vaccines:
In terms of the number of people vaccinated, J&J’s vaccine trails behind other vaccines authorized for use in the U.S. Approximately 367 million Americans have received a Pfizer shot, and over 232 million have received a dose of Moderna’s vaccine.
On the other hand, only about 19 million of the total shots administered in the U.S. since early 2021 were made by J&J.
Issues Surrounding J&J’s Vaccine:
The J&J vaccine was plagued by controversy since it entered the market in February 2021, with more than 2.1 million mRNA shots already having been administered. These shots, produced by Pfizer and Moderna, set a high standard that J&J found difficult to match.
The controversy further intensified when a rare but severe blood clotting disorder called thrombosis with thrombocytopenia syndrome (TTS) was linked to the vaccine.
Symptoms of TTS typically appear between four and 42 days after vaccination, leading the FDA and the Centers for Disease Control and Prevention (CDC) to pause administering the vaccine temporarily.
Despite reassurances from both agencies that the vaccine was safe and effective in preventing COVID-19, the damage was done. Public trust in the vaccine suffered, leading to a drastic reduction in demand.
The situation was exacerbated when a Baltimore-based manufacturing plant ruined approximately 15 million doses due to cross-contamination with the AstraZeneca vaccine.
Public Perception and the Future:
The reputation of the J&J vaccine suffered significantly despite the lift of the recommended pause by the federal government. Its single-dose nature became the object of ridicule, with comedy superstar Dave Chappelle even making a joke about it.
However, it’s important to note that the J&J vaccine was initially considered a game-changer for people experiencing homelessness and others who may have had difficulty accessing the second dose of the Moderna or Pfizer vaccines.
In December 2021, the CDC’s panel of vaccine experts recommended the Moderna and Pfizer mRNA vaccines over the J&J vaccine due to increasing reports of TTS. As of May 2022, the FDA restricted the use of the J&J vaccine to adults aged 18 and older who were unable to receive an mRNA vaccine due to allergy or unavailability.
Conclusion:
The revocation of the FDA authorization for the Johnson & Johnson COVID-19 vaccine marks a significant moment in the ongoing battle against the pandemic.
This situation underscores the importance of rigorous safety measures and transparency in vaccine development and distribution. The current scenario also underlines the necessity for effective public communication strategies to ensure public trust in vaccinations remains high.
In the face of dwindling public enthusiasm for vaccines, health organizations and governments worldwide must redouble their efforts to disseminate accurate information and ensure that as many people as possible are vaccinated. Only by doing so can we hope to bring an end to this devastating global pandemic.
References:

Trending Stories
Sister Regina Liu: Empowering Health Through Acupuncture

Sister Regina Liu: Empowering Health Through Acupuncture
In the bustling world of healthcare, Sister Regina Liu stands out as a beacon of holistic healing. Her journey into the world of acupuncture is not only inspiring but also transformative for the countless individuals she has treated.
Through her dedication, Sister Regina has brought traditional Chinese medicine to the forefront, offering an alternative and complementary approach to modern medical practices.
The Journey of Sister Regina Liu
Sister Regina Liu’s path to becoming a renowned acupuncturist began with her deep-rooted interest in holistic health. Born into a family that valued traditional Chinese medicine, Sister Regina was exposed to the benefits of acupuncture from a young age. Her early fascination turned into a lifelong passion as she pursued formal education and training in the field.
Acupuncture: Bridging Ancient Wisdom and Modern Health
Acupuncture, a practice with origins in ancient China, involves inserting thin needles into specific points on the body to balance the flow of energy or “qi.” Sister Regina Liu has mastered this ancient art, using it to address a wide range of health issues.
From chronic pain to stress management, her expertise has provided relief to many who had exhausted conventional treatment options.
Impact on Community Health
Sister Regina’s impact extends beyond individual treatments. She has been instrumental in educating the community about the benefits of acupuncture, breaking down misconceptions, and making the practice more accessible.
Her workshops and seminars have enlightened many about the holistic approach to health, emphasizing the interconnectedness of body, mind, and spirit.
Success Stories and Testimonials
The success stories of Sister Regina’s patients are a testament to her skill and dedication. Many individuals who had lost hope found solace in her treatments.
For instance, Maria, a long-time sufferer of migraines, experienced significant relief after just a few sessions with Sister Regina. Her story is just one of many that highlight the transformative power of acupuncture under Sister Regina’s care.
Challenges and Triumphs
Like any journey, Sister Regina’s path was not without challenges. Integrating acupuncture into mainstream healthcare faced resistance initially.
However, her perseverance and the undeniable results of her treatments gradually won over skeptics. Today, Sister Regina is not only respected in the field of acupuncture but also in the broader medical community.
The Science Behind Acupuncture
While acupuncture is rooted in ancient practices, modern science has begun to unravel the mechanisms behind its effectiveness. Studies have shown that acupuncture can stimulate the release of endorphins, the body’s natural painkillers, and improve blood circulation.
These scientific validations have further cemented acupuncture’s place in contemporary healthcare, thanks in part to advocates like Sister Regina Liu.
Acupuncture in Modern Healthcare
Sister Regina’s work exemplifies how traditional practices can complement modern medicine. Hospitals and clinics increasingly incorporate acupuncture into their treatment plans, recognizing its benefits in pain management, mental health, and overall well-being. This integration signifies a broader acceptance and understanding of holistic health practices.
Future Vision
Looking ahead, Sister Regina Liu envisions a future where acupuncture and traditional Chinese medicine are fully integrated into the global healthcare system. She continues to advocate for research, education, and policy changes that support the inclusion of holistic practices in mainstream medicine.
How to Get Started with Acupuncture
For those new to acupuncture, Sister Regina offers practical advice on getting started. She recommends finding a certified acupuncturist, understanding the treatment process, and maintaining an open mind. Her guidance helps demystify acupuncture, making it more approachable for newcomers.
Conclusion
Sister Regina Liu’s journey in empowering health through acupuncture is a remarkable tale of dedication, resilience, and success. Her contributions have not only alleviated individual suffering but also enriched the broader understanding of holistic health. As acupuncture continues to gain recognition, Sister Regina’s legacy will undoubtedly inspire future generations of healers.
FAQs
1. What conditions can acupuncture treat?
Acupuncture can address various conditions, including chronic pain, migraines, stress, anxiety, digestive issues, and more. It is also used to support overall wellness and balance.
2. Is acupuncture safe?
Yes, when performed by a certified and experienced acupuncturist, acupuncture is safe. It involves using sterile, single-use needles and adhering to proper hygiene practices.
3. How many sessions are needed to see results?
The number of sessions varies depending on the condition and individual response. Some may experience relief after one session, while others may need multiple treatments.
4. Does acupuncture hurt?
Acupuncture needles are very thin, and most people feel minimal to no discomfort. Some may feel a slight tingling or warmth at the needle site.
5. How do I find a qualified acupuncturist?
Look for acupuncturists who are certified by recognized professional organizations and have positive patient reviews. Personal recommendations and consultations can also help in making an informed choice.
References
Trending Stories
In 2 Shape Gym Unveils Major Expansion in Stourport
Trending Stories
9 Reasons Why In-Person Friendships Are Irreplaceable
-

 Trending Stories1 year ago
Trending Stories1 year agoCDC: 1 in 4 Americans Still COVID-Free by End of 2022
-

 Health5 years ago
Health5 years agoMeghan Trainor Shares Motivational New Song ‘Blink’
-
 Health2 years ago
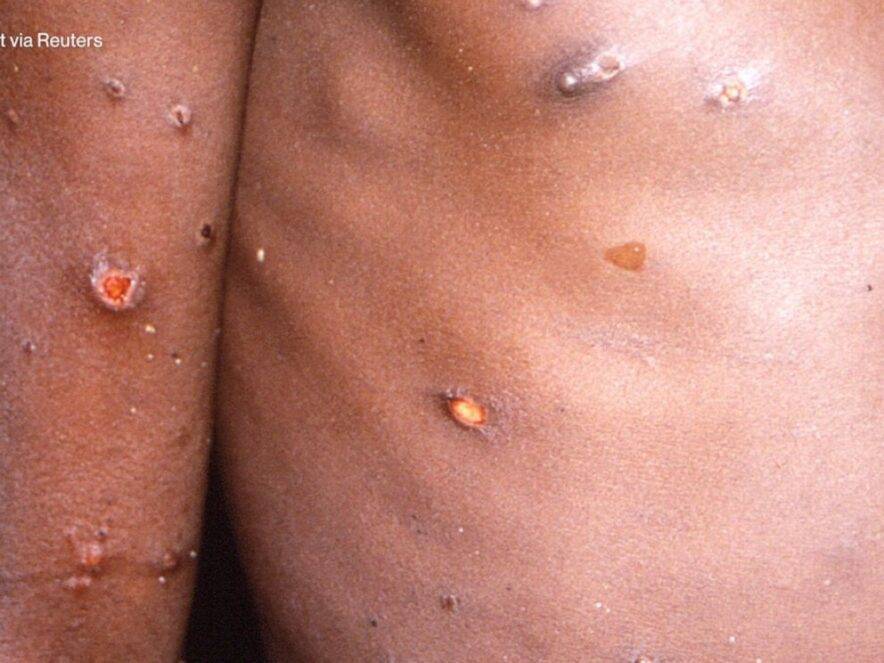
Health2 years agoHow Long Does Monkey Pox Last Before It Surfaces in the Body?
-

 Health2 years ago
Health2 years agoWhat Causes Swollen Body? Understanding Edema and its Triggers
-

 Health3 years ago
Health3 years agoNutrition and the Importance of a Fitness Program – 3 Things to Know
-

 Health3 years ago
Health3 years ago5 Weird Reasons Why Pimples Disappear After Marriage
-

 Health3 months ago
Health3 months agoHow Do Pawpaw Seeds Support Cardiovascular Health?
-

 Health2 years ago
Health2 years agoHealth Benefits Of Pawpaw Seed? 7 Things To Know